
Well, that should be a pretty straightforward calculation right now. and so if our total pressure is 700 and 37 mm of Mercury times 78. So if 70% of dry air is nitrogen than 78 of the pressure is from nitrogen. And so if volume and temperature constant, then pressure is proportional to molds. The only other thing we're told in this is that 78% of dry air is nitrogen. So whatever gasses are making up air, we know that if we add up the partial pressures of each of those, then we get the total pressure. So Dalton's law says the total pressure is equal to the sum of the partial pressures. Otherwise, this problem becomes really tricky. And so we've got to assume in this problem that volume and temperature constant. And then we're also going to be looking at the fact that pressure is proportional to moles when volume and temperature are constant.

Helium gas, He, at 22 degrees C and 1.00 atm occupied a vessel whose.
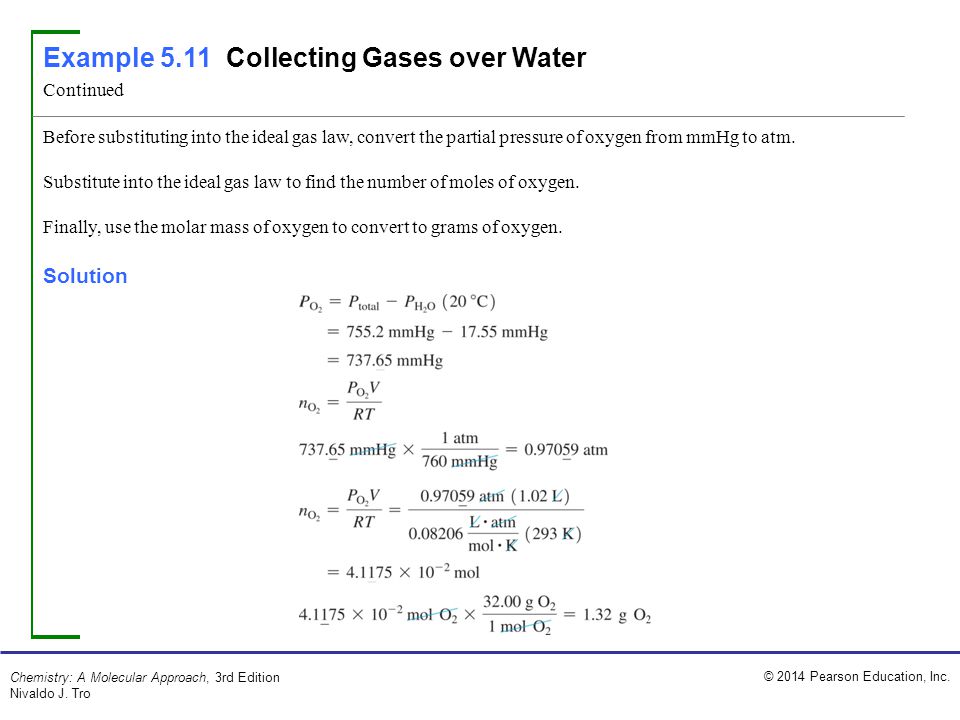
#737 mmhg to atm full
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types.So in this problem, we've got kind of a combination of principles that we're using here, we're going to be using dalton's of partial pressure. at a temperature of 17 C and a pressure of 737 mm Hg. 5420 0.6218 The Bunsen coefficients were. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. Coefficient Coefficient 1 o/cm (STP) cm' atm' L/cm3 cm- 282 - 80 5. We count on that the closed vessel has 1L of volume. Although not an SI unit, the millimetre of mercury is still routinely used in medicine, meteorology, aviation, and many other scientific fields. The partial stress of H2 is 737.47 mmHg Let's observe the Ideal Gas Law to find out the whole mols. The unit is named after Blaise Pascal, the eminent French mathematician, physicist and philosopher.Ĭonversion calculator for all types of measurement units. A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly 133.322 387 415 pascals. The pascal (symbol Pa) is the SI unit of pressure.It is equivalent to one newton per square metre. Gas Laws Practice Problems KEY Boyle’s Law What pressure will be needed to reduce the volume of 77.4 L of helium at 98.0 kPa to a volume of 60.0 L P1V1 P2V2 P2 P1V1V2 P2 98 kPa x 77.4 L60 L P2 126 kPa A 250.0mL sample of chlorine gas is collected when the b. The definition of a pascal is as follows: Although not an SI unit, the millimetre of mercury is still routinely used in medicine. 970 ATM A gas occupies 12.3 liters at a pressure of 40.0 mmHg what is the volume when the pressure increases to 60. The SI prefix "kilo" represents a factor of A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly 133.322 387 415 pascals. On a rainy day a barometer reads 737 mmhg Convert this value to atmospheres.

This small difference is negligible for most applications outside metrology. Calculations Twater(K) 1/ Twater(1) Pair(mm Hg) Pwater(mm Hg) Pwater(atm). The difference between one millimeter of mercury and one torr, as well as between one atmosphere (101.325 kPa) and 760 mmHg (101.3250144354 kPa), is less than one part in seven million (or less than 0.000015%). Data Collection Barometric pressure, Pear (mm Hg) 737,3 Temperature and Volume. How do you convert a 50 micro-amp DC meter to a 100 milli-amp meter.

The relationship between the torr and the millimeter of mercury is: Use this formula to convert from Fahrenheit to Celsius: ° C (° F &minus 32) × 5⁄9. The decimal form of this fraction is approximately 133.322368421. Therefore, 1 Torr is equal toġ01325/760 Pa. The torr is defined as 1/760 of one standard atmosphere, while the atmosphere is defined as 101325 pascals. Task: Convert 975 torrs to atmospheres (show work) Formula: Torr ÷ 760 atm Calculations: 975 Torr ÷ 760 1.28289474 atm Result: 975 Torr is equal to 1. The millimeter of mercury by definition is 133.322387415 Pa (13.5951 g/cm3 × 9.80665 m/s2 × 1 mm), which is approximated with known accuracies of density of mercury and standard gravity. You can view more details on each measurement unit: mmHg or psi The SI derived unit for pressure is the pascal. We assume you are converting between millimeter of mercury 0 ☌ and pound/square inch. STP: Standard Temperature and Pressure: 273 K and 1 atm (or. TEMPERATURE: Kelvin, K, which is oC + 273.
KPa to mm Hg, or enter any two units below: Enter two units to convert From: How many mmHg in 1 psi The answer is 51.714924102396. PRESSURE: atmospheres or mm Hg 1 atm 760 mm Hg. You can do the reverse unit conversion from


 0 kommentar(er)
0 kommentar(er)
